

It is recognized that we are ill equipped to deal with the emergence of a 1918-style pandemic that immediately causes high mortality and transmits rapidly. Improvements in dealing with antigenic drift will ensure the ability to rapidly implement a pandemic plan. Pandemic plans include improved ability to respond to antigenic drift on an annual basis with increased surveillance, rapidity of preparing vaccines, and the utilization of available antiviral drugs.

Planning for the next human pandemic has been done in many countries and is promoted by the World Health Organization. It is considered inevitable that there will be another human influenza pandemic and as time passes the likelihood increases. Thus the long-term perspective for influenza is the development of improved control strategies. The natural reservoirs of influenza viruses in aquatic birds throughout the world means that influenza is not eradicable. Webster, in Encyclopedia of Virology (Second Edition), 1999 Future PerspectivesĬontinuing antigenic drift and irregular antigenic shift are neither predictable nor preventable. Meanwhile the M2 protein disrupts host autophagy and disrupts activation of PKR. The PB1 and PB2 components of the IAV polymerase are able to interfere with the function of MAVS, which results in disruption of RIG-I signaling and limits IFN production and the creation of an antiviral cellular state. The IAV polymerase can also inhibit host cell translation by stealing the cap complexes from host mRNAs and therefore stopping recruitment of the ribosome. This serves to globally suppress immune activity and allows the virus to dominate usage of the host translational machinery. NS1 also inhibits host gene expression by interfering with mRNA processing events and by disrupting the function of the translation initiation factor eIF4B. It also possesses RNA binding capabilities and can therefore act in direct competition with TLRs and RIG-I to inhibit the detection of viral RNA. For example, it interacts with a range of proteins involved in IFN-related immune defenses and neutralizes their activity. The viral protein NS1 is a key mediator of IAV innate immune evasion. As with many viral infections, particularly the most successful ones, IAV has a wide repertoire of innate immune evasion strategies. These two processes are critical immune evasion tools, but function against the adaptive and not the innate immune response. The processes of antigenic drift and shift have been briefly described earlier. Monie, in The Innate Immune System, 2017 4.4.4 Evasion of the Innate Immune Response by Influenza A Virus Influenza pandemics are listed in Table 25-13. Seven reassortments or antigenic shifts in type A influenza have been documented in the last 115 years. It contains genes from pigs normally found in Europe and Asia, avian–swine influenza genes, and human influenza genes. The virus that caused the 2009 pandemic influenza (type A H1N1) is a quadruple reassortment virus. Within an infected porcine cell, reassortment of genetic material from both viruses creates a new, hybrid virus. For example, pigs carry an endemic strain of influenza and can be infected with both human and avian influenza strains.
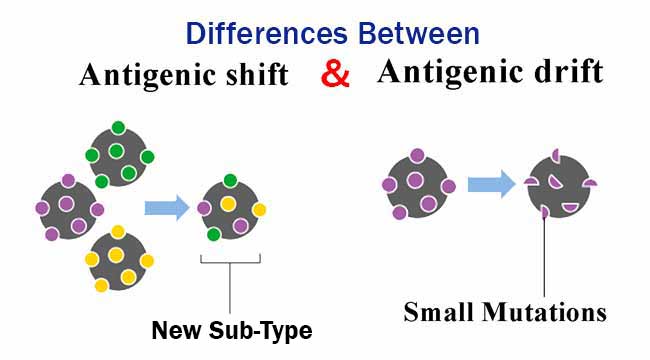
It occurs when two viruses simultaneously infect the same animal. Viral reassortment is a more complex form of antigenic shift. Worldwide, the death toll was estimated at 50 million. The type A H1N1 pandemic flu killed 500,000 people in the United States. Antigenic shift can be the result of a direct jump from an unknown animal strain to humans or a reassortment of two or more influenza viruses within the same cell.Įvidence suggests that the 1918 influenza pandemic was the result of a direct jump from pigs to humans. In some cases, a 50% change occurs in the hemagglutinin structure. In Immunology for Pharmacy, 2012 Antigenic ShiftĪntigenic shift occurs when a radical and abrupt change in influenza type A virus hemagglutinins occurs.


 0 kommentar(er)
0 kommentar(er)
